1. Chloramine is a combination of chlorine and ammonia.
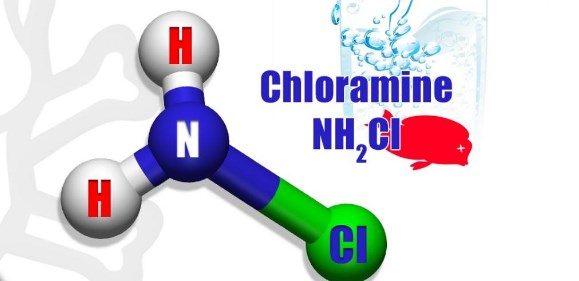
2. Chloramine exists in three different forms. This is why it is referred to in plural (chloramines). These are three forms are
- Monochloramine -NH2Cl
- Dichloramine- NHCCl2
- Trichloramine -NHCL3
3. Chloramine molecules are always in flux charging from one of the three forms to the next depending on the acidity level of the water.
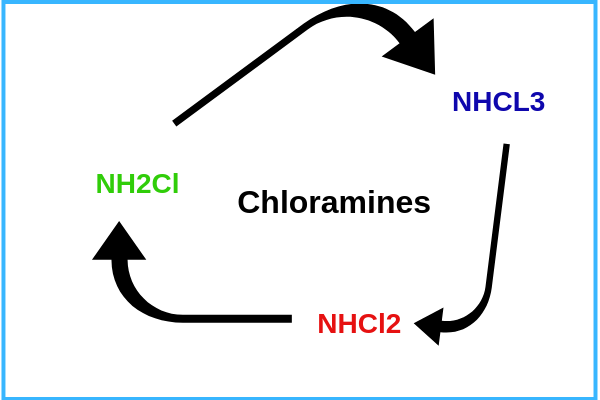
3. All three forms of chloramines are harmful to human health, but the most dangerous being Trichloramine followed by Dichloramine.
4. Chloramine is used as a secondary disinfectant after the water has already been disinfected to help protect water from re-infection as it travels through pipes into homes. The primarily disinfectant that is used in most cases is chlorine. In some cases, chlorine is used both as the primary and the secondary disinfectant.
5. A survey by the EPA estimated that 68 million Americans drink water treated with chloramines.

6. Chloramine is more stable than chlorine; thus, it survives for a longer period of time while the water goes through pipes. This is unlike chlorine when which dissipates quickly.
7. THMs are the byproducts that are formed when chloramines combine with organic matter. Chloramine produces a lower level of disinfection byproducts compared to chlorine.
8. The best way to prevent a disinfectant by-product is to fast remove the organic matter before chlorine or chloramines is added to water.

9. Chloramine produces lower levels of disinfectant byproduct compared to chlorine.
10. EPA has enacted stringent rules on the amount of this disinfectant byproduct that is allowable, and as a result, more and more cities are replacing chlorine with chloramine as a disinfectant.
Features of Chloramines
11. Compared to chlorine, chloramine does not evaporate quickly unlike chlorine which evaporates easily.
12. Chloramine remains in the water distribution system for a longer time.
13. Although a carbon filter can be able to filter chloramine, it requires more contact time with the filter for it to be absorbed.
14. Boiling water does not help to get rid of chloramines.
15. Research on the safety of chloramine is inconclusive.
History of chloramines
16. Chloramine has been used as water treatment disinfectant since the begging of the 20th century.
17. The first cities to use chloramine are Denver which started using it in 1918 and Boston which adopted it in the 1930s.
18. During World War 1, cities that were using chloramine to disinfect water were forced to stop using it because there was a general shortage of ammonia.
19. According to EPA, there are no conclusive studies that prove that exposure to chloramine can lead to cancer in humans.
Negative effects of chloramines
20. Inhaling chloramine can lead to development or make worse existing breathing problems. When someone inhales chloramine fumes they result in chest congestion. It can result in shortness of breath and asthma.
21. Exposure to chloramine occurs more from showering with chloramine water than drinking water.
22. When you inhale chloramine fumes they enter the bloodstream directly through the lungs. When monochloramine comes into contact with hemoglobin, it impairs the ability of red blood cells to transport oxygen throughout the body.
23. Long term exposure to chloramine increases the chance of becoming sick. Its effects might not be immediately noticeable.
24. Some studies have shown increased cases of pneumonia in communities that use chloramine.
25. When you inhale chloramines fumes, they damage the lung mucosa. When your mucosa is destroyed, you are more prone to developing allergies.
26. When the Skin is exposure to chloramine, it can trigger skin conditions such as rashes, itching, dry skin, and pigmentation.
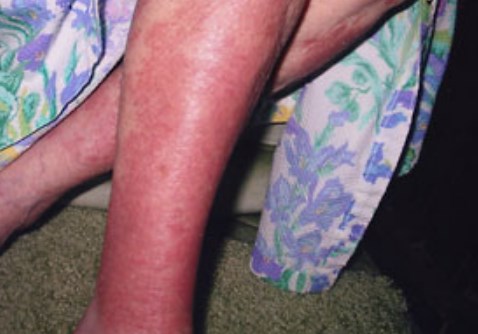
27. Chloramine causes the lips and mouth to become dry and the inflammation of the throat.
28. If you have skin conditions such as eczema and acne, chloramine will make them worse.
29. Digestive disorders such as ulcers can be aggravated when you drink water that is rich in chloramines because it damages digestive mucosa.
30. There is a high chance of suffering from ammonia poison especially for people who suffer from kidney and/or liver failure. For this reason, water that is used for dialysis undergoes extensive filtration to remove chloramine.
31. Although chloramine is last longer in water than chlorine, it’s not as effective in killing bacteria. It increases the risk of being exposed to pathogens and bacteria, therefore, increasing the risk of infection especially for people with a weak immune system.
32. Studies have shown that your genetic makeup can determine if you are more prone to suffering from ammonia toxicity. In fact, in some places, the cases of ammonia toxicity are very high because of the gene pool that exists among the population.
Environmental Effects of chloramine
33. When a large volume of chloramine water is discharged into the freshwater ecosystem it has a negative effect because it kills aquatic life.

34. Although chlorine is also poisonous to aquatic life, it dissipates more quickly. On the other hand, chloramine does not dissipate quickly.
35. Marine life such as fish cannot survive in chloramine water because it gets into their bloodstream and kills them.
36. Some of the disinfection byproducts that are formed in the water pose a threat to the environment because they are poisonous.
37. Canada has deemed chloramine “toxic” in its Environmental Protection Act (CEPA 1999).
38. Chloramine increases the risk of lead poison because chloramine water is more corrosive. It can seep lead from the plumbing materials.
39. Chloramine can trigger rubber corrosion which leads to faster depreciation of plumbing materials.
40. Businesses that must filter chloramine from the water before using the water incur additional costs because chloramine is a stubborn contaminant to remove. Example of business that needs to remove chloramine includes pharmaceutical’s food and beverage companies.
Filtration of chloramines
41. Filtration of chloramine is more complicated than the filtration of chlorine. Although there is an urban myth that carbon filters don’t remove chloramine. Carbon filters are able to filter chloramines, but they do require more contact time for carbon to filter chloramine.
42. the most effective method of filtering chloramines is by use of a combination of carbon and reverse osmosis.
43. Showerhead filters that use carbon filtration are not very effective in removing chloramines because there will not be enough contact period between chloramine and carbon for absorption to take place.
44. A whole-house system that uses a combination of carbon filters and reverses osmosis is ideal for dealing with chloramines.
45. Vitamin c shower heads are more effective in neutralizing chloramine than carbon-based showerheads. Vitamin-C acts as a reducing agent.
46. Faucet filter such as over the counter or under the counter filter s is only able to handle low flow filtration of chloramines.
47. Boiling water does not remove chloramine.
48. Distillation is not an option when it comes to removing chloramine because the boiling point of chloramine is lower than that of water.
49. In an industrial setting, the most common methods of removing chloramine are the addition of reducing agents such as sulfite compounds, hydrogen peroxide, and ascorbic acid.
Finally, if you are looking to remove chloramine from shower water, check out of our comprehensive review of the best shower head filters for chloramine
ABOUT LEON SMITH
 Leon is a 27-year-old blogger from Mauritius who is currently studying for a Masters’s degree in chemical and processing Engineering at the University of Eldoret in Kenya.
Leon is a 27-year-old blogger from Mauritius who is currently studying for a Masters’s degree in chemical and processing Engineering at the University of Eldoret in Kenya.